HTML
-
甲基菲(MP)与苯基菲(PhP)广泛分布于烃源岩和石油中,是两类重要的多环芳烃化合物。其中对甲基菲的检测、分布、成因及地球化学应用的研究较多,其相关的地球化学指标已广泛应用于原油和烃源岩有机质的成熟度判识[1⁃4]。然而,对苯基菲的研究相对较少。Hawley-Fedder et al.[5]于1984年首次在聚苯乙烯燃烧后的产物中检测发现1-苯基菲(1-PhP)。随后,苯基菲系列化合物在烟煤经过氧化脱羧反应后的产物[6]、煤焦油沥青热解的挥发物[7]、沉积的灰尘[8]、沉积有机质[9⁃12]及原油[13]中均被检测报道。由此可见,苯基菲广泛分布于工业产物、环境及地质体中。
前人研究认为,苯基取代多环芳烃主要具有两种来源。工业产物和环境中的苯基多环芳烃主要是生物质或者其他多环芳烃高温燃烧或者热解的产物[6⁃8,14⁃15],而地质体中的苯基多环芳烃主要通过岩石成岩/后生阶段发生的氧化作用形成[9,16⁃18]。其中关于沉积有机质中苯基菲的形成及转化机理,Marynowski et al.[18]认为苯基菲是地球化学成岩作用的产物,由其他化合物分子结构经过重排作用形成,其没有明确的生物前驱物。Rospondek et al.[9]认为苯基菲是菲的自由基苯基化和苯基迁移作用的产物,在成岩作用阶段菲通过自由基苯基化作用优先形成4-PhP、1-PhP及9-PhP,而在后生作用阶段其分布主要由热力学控制,随着成熟度的增加苯基菲通过苯基迁移作用转化为热稳定性更大的异构体。Grafka et al.[12]认为沉积岩中的苯基菲是苯基自由基与有机质之间反应的产物。由此可见,苯基菲的来源与沉积有机质的生物先质无明显关系,没有明确的继承性,其为成岩作用/后生作用过程中不同化合物之间反应及转化的产物。
目前,关于苯基菲地球化学意义的研究主要集中在成熟度判识方面。Rospondek et al.[9]在沉积岩中检测到苯基菲系列,并提出成熟度参数(2-+3-PhP)/[(2-+3-PhP)+(4-+1-+9-PhP)]。然而,Li et al.[11]在研究辽河盆地湖相烃源岩成熟度时,发现该参数的应用效果并不理想。目前,该指标在有机质成熟度判识的应用还存在争议。另外,该参数仅为定性评价成熟度的相对指标,尚未建立可应用于定量评价烃源岩和原油成熟度的关系式。鉴于存在上述问题,本文选取准噶尔盆地和鄂尔多斯盆地煤,共计26件。采用标样相对保留时间和标准保留指数对比的方法,系统、明确地鉴定出苯基菲系列。通过量子化学计算方法,精确计算了该系列的热稳定性大小,探讨了成熟度和沉积环境对其分布的影响,初步构建可应用于定量评价烃源岩和原油成熟度的指标。通过与甲基菲分布和热稳定性大小的对比,探讨了苯基菲指示有机质热演化程度的化学机理。
-
准噶尔盆地位于中国西部,发育在前寒武系与前石炭系双重基地之上[19],面积为13.5×104 km2。侏罗系在盆地内分布广、厚度较大,是一套湖沼相的含煤碎屑沉积建造,富含煤层及炭质泥岩。本研究选取准噶尔盆地侏罗系煤12件,成熟度较低,镜质组反射率(Ro)为0.46%~0.76%,处于未熟—低熟阶段,干酪根类型为II~III型,姥鲛烷/植烷比值(Pr/Ph)介于3.66~14.66。
鄂尔多斯盆地位于华北地台的西端,为多旋回叠合克拉通盆地,面积为37×104 km2,其中古生界沉积岩分布面积为25×104 km2[20]。盆地内石炭系—二叠系地层主要沉积于海陆过渡相,发育多套煤层、煤系页岩、砂岩,还含有部分灰岩。本研究选取鄂尔多斯盆地石炭系—二叠系煤14件,成熟度较高,大部分样品Ro介于0.96%~1.88%,处于成熟—高成熟度阶段,干酪根类型为II~III型,Pr/Ph为0.49~2.84。
苯基菲共有5个异构体,分别为1-苯基菲(1-PhP)、2-苯基菲(2-PhP)、3-苯基菲(3-PhP)、4-苯基菲(4-PhP)及9-苯基菲(9-PhP)。本研究从挪威Chiron科学公司购置了1-、2-、3-及9-苯基菲,用于样品中该系列化合物的鉴定。
-
先将煤粉碎至80目以下,然后用400 mL的二氯甲烷:石油醚(93∶7,体积比)的混合液,采用索氏抽提法萃取煤中可溶有机质,用石油醚析出并除去沥青质后,再依次用石油醚、二氯甲烷:石油醚(2∶1,体积比)混合液、二氯甲烷:甲醇(93∶7,体积比)混合液,分别洗脱出饱和烃馏分、芳烃馏分及非烃馏分。标样和芳烃馏分的色谱—质谱分析仪器为Agilent6890 GC⁃5975i MS色谱—质谱联用仪,配置HP-5MS色谱柱(60 m×0.25 mm×0.25 μm)。具体的仪器条件为:色谱柱加热箱初始预热温度设置为80 ℃,恒定温度持续1 min,后以3 ℃/min的速率加温,升至310 ℃,然后保持恒温16 min。质谱仪采用电子轰击(EI)方式,电离能量为70 eV,数据采集方式为全扫描和选择离子检测,质量扫描范围50~600 Da。
煤的镜质组反射率测定仪器为ZEISS Axio Imager 2生物显微镜,外接MPV-SP显微分光光度计,反射率测量量程为0.1%~10.0%,分辨率大小为0.01%,测量过程严格执行SY/T 5124—2012行业标准,每个样品随机反射率测定点数(有效测点数)都大于60个,最后以平均值输出为样品的实测反射率,保证测得的Ro数据准确、可靠。
在不同仪器、不同实验条件下,同一个化合物在色谱柱的保留时间(tR)往往不同。标准保留指数(I)可以反映某个分子标志物在一定实验分析测试条件下在色谱柱上的流出特征,便于不同实验室之间进行对比,常采用保留指数系统进行化合物鉴定,某个多环芳烃分子标志物的标准保留指数具体的计算方法和公式参照文献[21]。
此外,本研究采用密度泛函理论(DFT)方法计算了苯基菲和甲基菲各异构体的热稳定性,获取了诸如:电子能(ΔE)、内能(ΔU)、焓(ΔH)、吉布斯自由能(ΔG)等热力学信息。DFT方法是非常成熟可靠的,在本课题组先前的研究中已获得了成功的应用[22⁃24]。本文所有的量子化学计算都是通过Gaussian 09软件包实现的,量子化学计算采用密度泛函B3LYP方法,具体基组为6-311++G(d,p)。
-
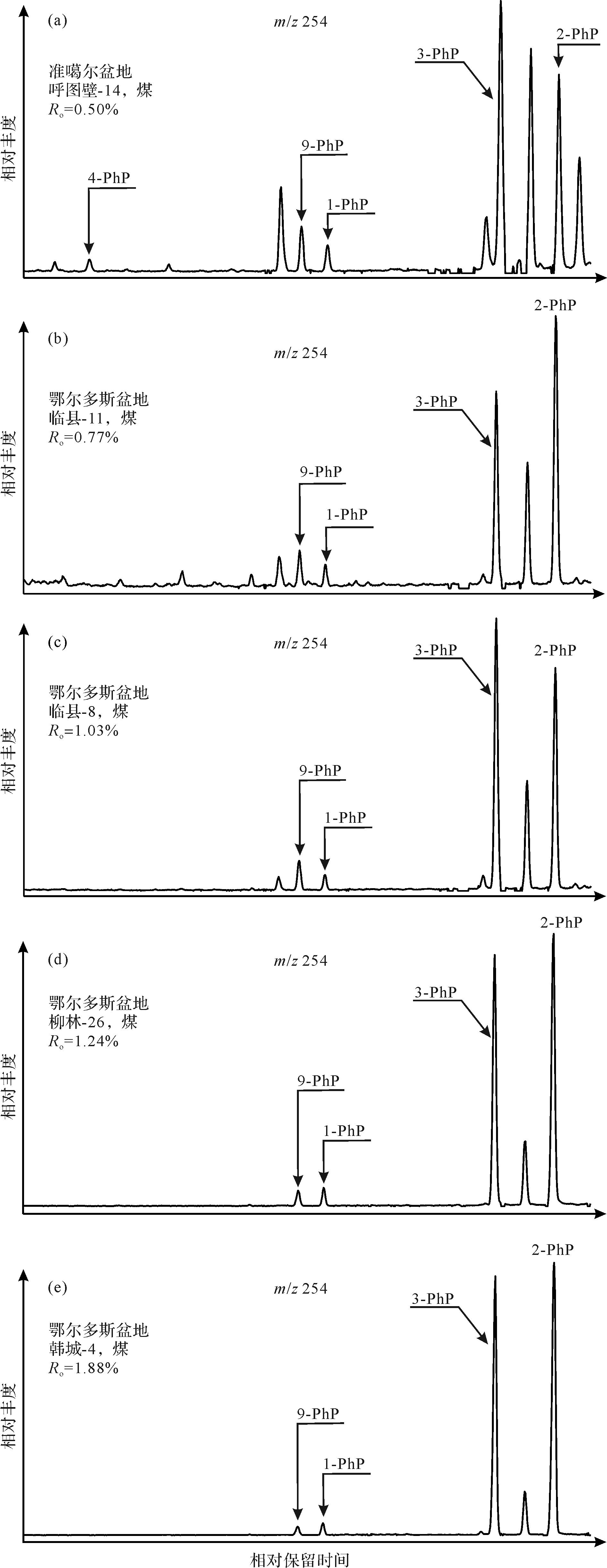
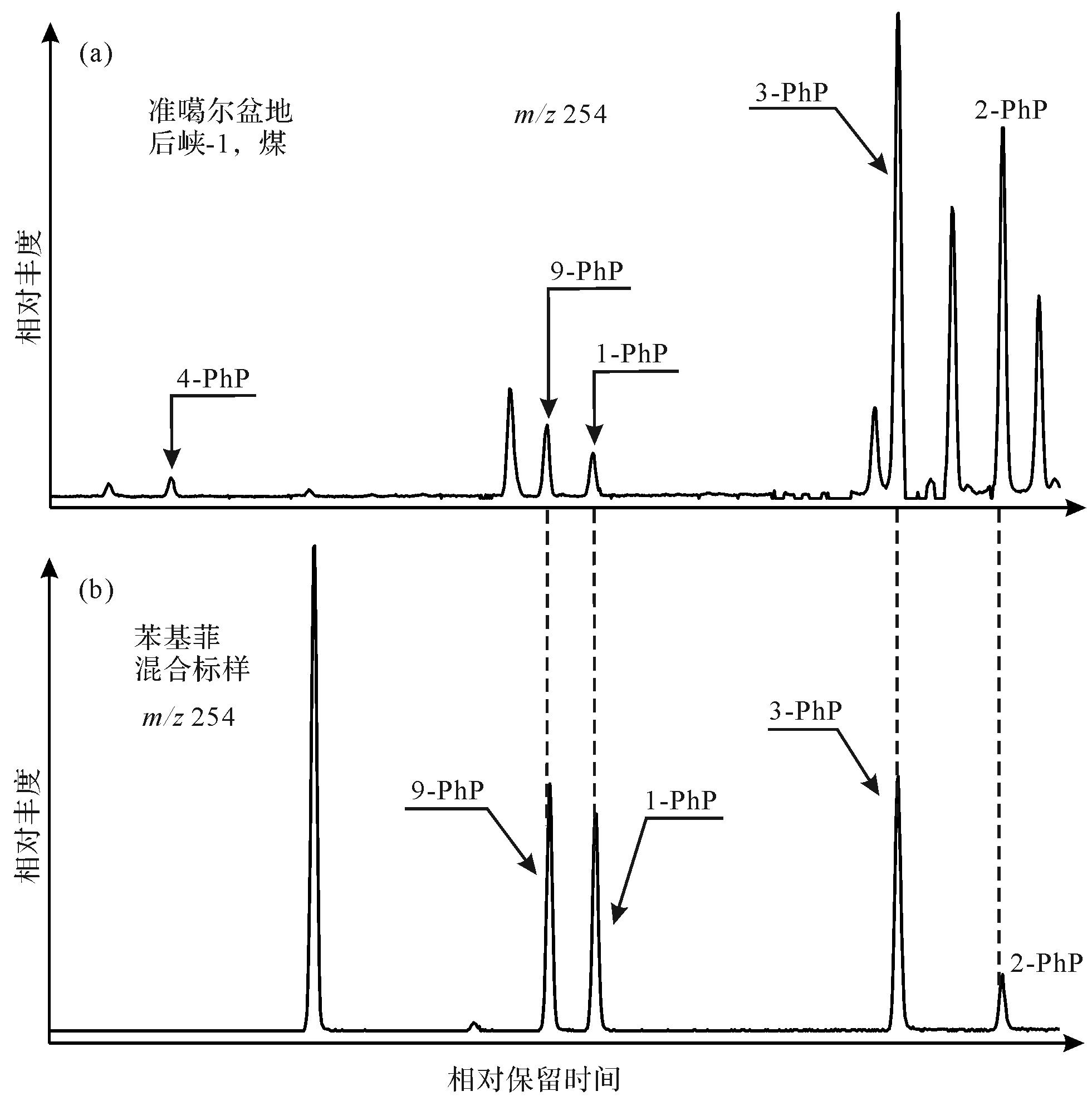
Rospondek et al.[9]采用标样相对保留时间对比的方法,首次在沉积有机质中鉴定出苯基菲系列,并计算了各异构体在色谱柱(HP-5MS)上的保留指数。随后,Li et al.[11]通过标样保留指数对比的方法,在湖相页岩中鉴定了该系列化合物,确定了其在芳烃馏分m/z 254质量色谱图中的流出顺序。对苯基菲的系统、准确地鉴定是探讨其油气地球化学应用的前提。本研究采用标样相对保留时间和标准保留指数对比的方法,明确鉴定出煤中的9-、1-、3-、2-苯基菲。通过保留指数系统,精确计算出苯基菲各异构体的保留指数(表1),同时鉴定出4-苯基菲。由此,确定了各异构体具体的流出位置和顺序:4-、9-、1-、3-、2-苯基菲(图1),结果与前人的结论相吻合。另外,本研究测定的苯基菲系列保留指数与前人的报道基本一致,差别都在±1.0指数单元以内,考虑到色谱—质谱实验条件的影响,认为该误差在合理的范围之内。因此,本文计算的苯基菲系列保留指数可以为其他研究人员在相同实验条件下(60 m,HP-5MS色谱柱,升温程序)对该类化合物的鉴定提供依据。
化合物 文献[9] 文献[11] 标样 煤,后峡-1 鉴定结果 I I tR I tR I 菲 300.00 300.00 40.239 300.00 41.615 300.00 菲 4-苯基菲 389.05 388.82 — — 59.974 388.22 4-苯基菲 䓛 400.00 400.00 60.789 400.00 62.425 400.00 䓛 9-苯基菲 405.63 405.08 61.633 404.86 63.184 404.48 9-苯基菲 1-苯基菲 407.82 407.33 62.025 407.12 63.576 406.79 1-苯基菲 3-苯基菲 422.87 422.07 64.602 421.97 66.180 422.14 3-苯基菲 2-苯基菲 427.64 428.07 65.495 427.11 67.076 427.43 2-苯基菲 苉 500.00 500.00 78.147 500.00 79.383 500.00 苉 -
为了对比苯基菲和甲基菲各异构体的热稳定性差异,通过量子化学计算的方法精确计算了两系列不同异构体的热力学性质,包括ΔE、ΔU、ΔH、ΔG等。表2显示,4-苯基菲的热稳定性最低,造成其在地质样品中很难被检测到。1-、9-苯基菲的稳定性较低,2-、3-苯基菲的热稳定性较高,这4个异构体普遍存在于地质体中。苯基菲系列热稳定性大小顺序为:2->3->9->1->4-PhP,与研究人员根据它们在不同成熟度样品中的分布而确定的热稳定大小相符合[9,11]。甲基菲与苯基菲系列各异构体的热稳定性大小顺序一致,为:2->3->9->1->4-MP,与前人通过量子化学计算的结果一致[25]。对比发现,苯基菲和甲基菲系列的相对热稳定性大小由取代位置决定,均为:C-2>C-3>C-9>C-1>C-4,而与取代基类型无关。Szczerba et al.[25]认为甲基菲系列热稳定性的差异主要是由空间位阻效应导致的,由于取代位C-1到C-9的空间位阻效应不同,造成各异构体几何构型产生不同程度的形变,导致热稳定性大小不同。
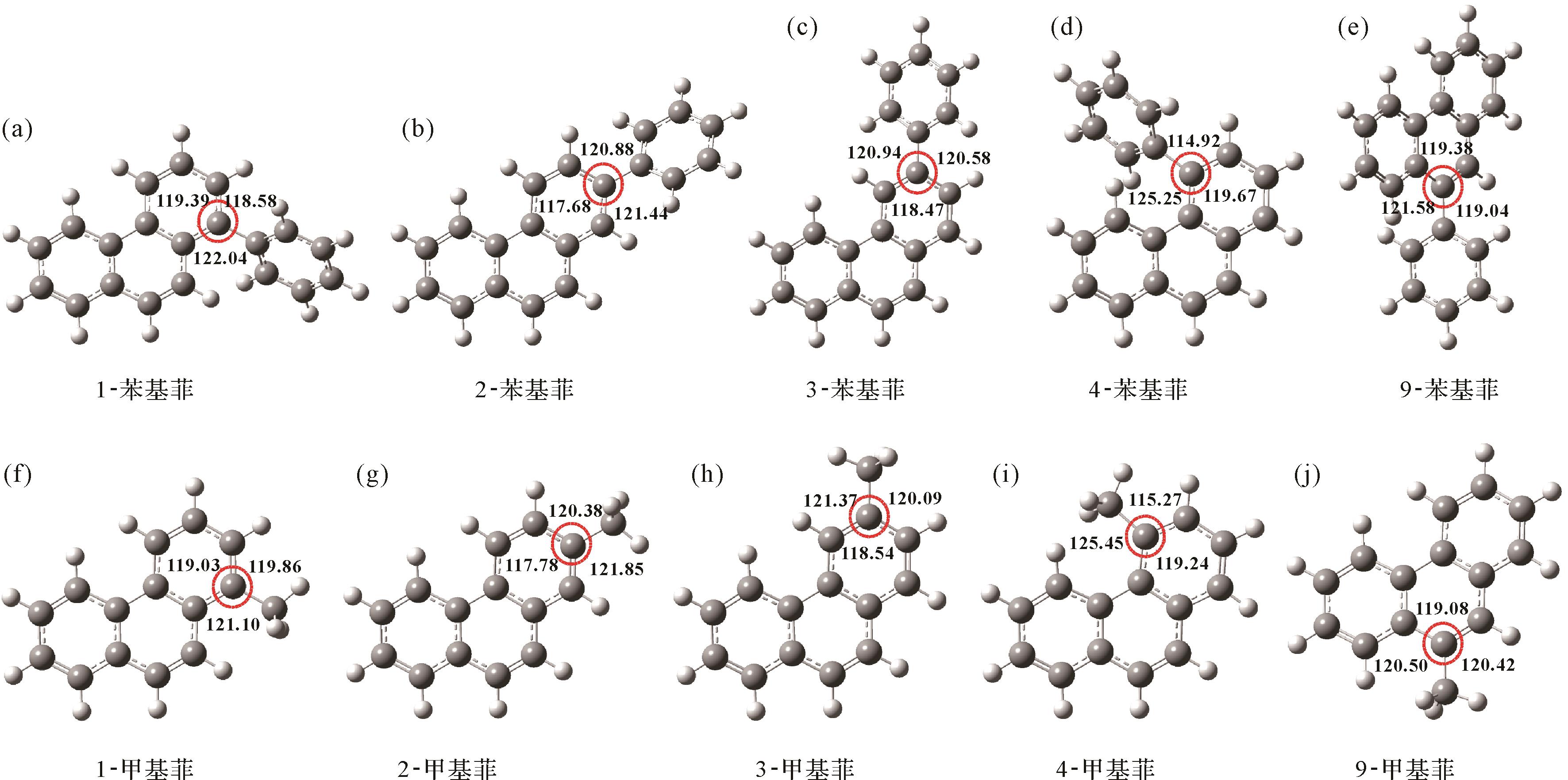
化合物 ΔE(kcal/mol) ΔU(kcal/mol) ΔH(kcal/mol) ΔG(kcal/mol) Debye(μ) 2-苯基菲 0.00 0.00 0.00 0.00 0.02 3-苯基菲 0.04 0.03 0.03 0.04 0.03 9-苯基菲 2.71 2.74 2.74 2.65 0.14 1-苯基菲 2.81 2.82 2.82 2.79 0.15 4-苯基菲 7.95 7.93 7.93 8.05 0.20 2-甲基菲 0.00 0.00 0.00 0.00 0.58 3-甲基菲 0.12 0.10 0.10 0.15 0.56 9-甲基菲 1.28 1.12 1.12 1.73 0.43 1-甲基菲 1.65 1.51 1.51 2.04 0.39 4-甲基菲 7.86 7.60 7.60 8.15 0.34 采用密度泛函理论计算方法,对苯基菲和甲基菲系列各异构体的分子几何结构进行了优化,确定了最优化条件下的键角。在理想状态下,苯基菲上的苯基与菲之间的夹角为120°,但由于不同取代位置具有不同的空间位阻效应,造成各异构体几何构型产生不同程度的形变,苯基与菲之间的夹角也会改变。如图2所示,苯基菲各异构体几何构型变形程度由大到小依次为:4->1->9->3->2-PhP,与苯基菲的热稳定性大小顺序相反,分子结构变形程度越大,热稳定性越低。与其类似,甲基菲各异构体几何构型变形程度由大到小依次为:4->1->9->3->2-MP,也与甲基菲系列的热稳定性大小顺序相反。同样,苯基菲和甲基菲系列的分子结构变形程度也由取代位置决定,均为:C-4>C-1>C-9>C-3>C-2,而与取代基类型无关。由此认为,苯基菲和甲基菲系列不同取代位置的空间位阻效应大小为:C-4>C-1>C-9>C-3>C-2,不同取代位置的空间位阻效应的不同造成了各异构体的热稳定性差异。
-
本次采集的煤中均检测出苯基菲系列化合物,图3展示了不同成熟度煤中苯基菲的分布特征,随着成熟度的增加,各异构体的相对丰度表现出规律性的变化,表明成熟度是控制该系列分布的重要因素。总体来看,大部分煤均具有较高含量的3-、2-苯基菲,以及较低含量的9-、1-苯基菲。然而,4-苯基菲仅以微量分布于成熟度相对较低的煤中(Ro<0.77%),在成熟度较高的样品中基本检测不到该化合物(图3),这与该化合物具有低的热稳定性有关。比较发现,相对于3-、2-苯基菲,9-、1-苯基菲的丰度随着成熟度的升高而逐渐降低(图3),原因可能是9-、1-苯基菲的热稳定性相对较低。所以,根据不同成熟度样品中苯基菲的分布特征,推测该系列的热稳定性大小顺序为:3-、2->9-、1->4-PhP,与量子化学计算的热稳定性大小顺序基本一致。
基于地化数据和量子化学计算的结果,以苯基菲系列异构体之间热稳定性的差异为基础,本文提出了苯基菲比值(PhPR=(2+3)-PhP/(1+9)-PhP)这一成熟度参数。从图4可以看出,苯基菲比值(PhPR)随着镜质组反射率(Ro)的增加而增大,两者表现出良好的线性正相关性,相关系数(R2)达到0.94,表明苯基菲比值是潜在的成熟度参数。另外,初步建立了通过苯基菲比值定量评价成熟度的换算公式:Rc=0.06×PhPR+0.32(Ro>0.4%),可应用于低熟—高熟原油和烃源岩(II~III型干酪根)的成熟度评价。
-
苯基菲比值能否成为有效的成熟度指标,还需要研究沉积环境对该参数的影响。沉积岩中有机显微组分能够反映生源类型,镜质组和惰质组分别由高等植物木质、纤维组织经过凝胶化作用和丝炭化作用形成,壳质组来源于高等植物类脂的膜质物或分泌物,腐泥组是藻类及其他低等水生生物腐泥化作用的产物[26]。前人研究认为,C29甾烷主要来自陆源高等植物,C27甾烷指示底栖藻类和浮游藻类的贡献[27⁃28]。本研究中准噶尔盆地和鄂尔多斯盆地煤中有机显微组分基本只含有镜质组和惰质组,两者之和占总有机显微组分的94.4%以上,壳质组含量极低(<5.6%),未观测到腐泥组。此外,煤中C29甾烷含量优势明显,而C27甾烷含量极低。由此可知,所研究煤中的有机质生源类型差异不大,主要为陆生高等植物。
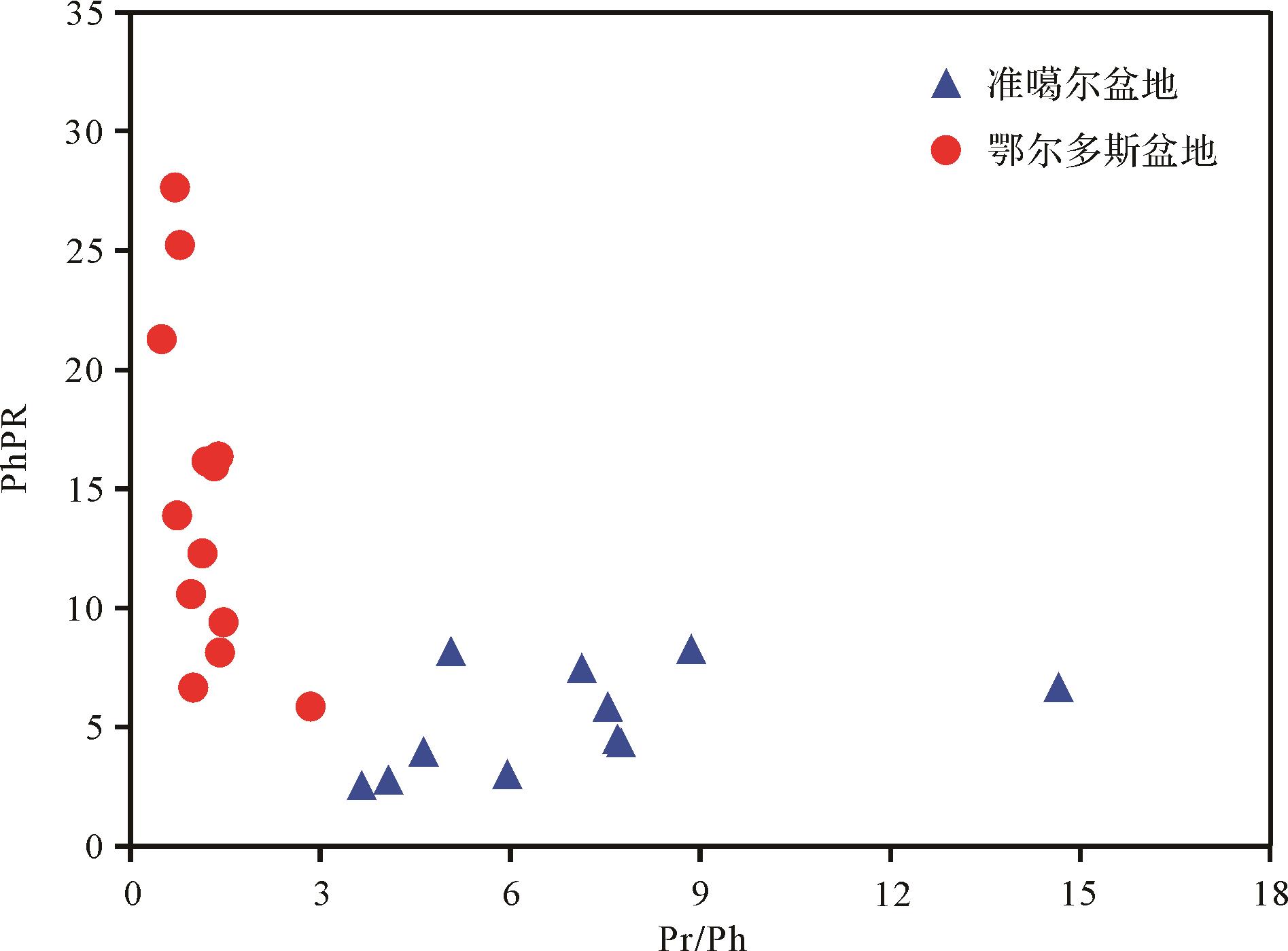
姥鲛烷/植烷比值(Pr/Ph)是广泛用于指示沉积环境氧化还原性的参数[28⁃29]。从苯基菲比值与姥鲛烷/植烷比值的关系图中可以看出(图5),两者没有明显的相关性。处于偏还原环境(Pr/Ph<2)的煤,PhPR变化范围很大,介于6.56~27.66。对于偏氧化环境(Pr/Ph>2)的煤,随着Pr/Ph的增加,PhPR基本保持不变,介于2.55~8.27。结果表明,沉积环境对PhPR的影响很小。所以,苯基菲比值可作为良好的成熟度参数,对于还原—氧化沉积环境的烃源岩和原油均适用。
-
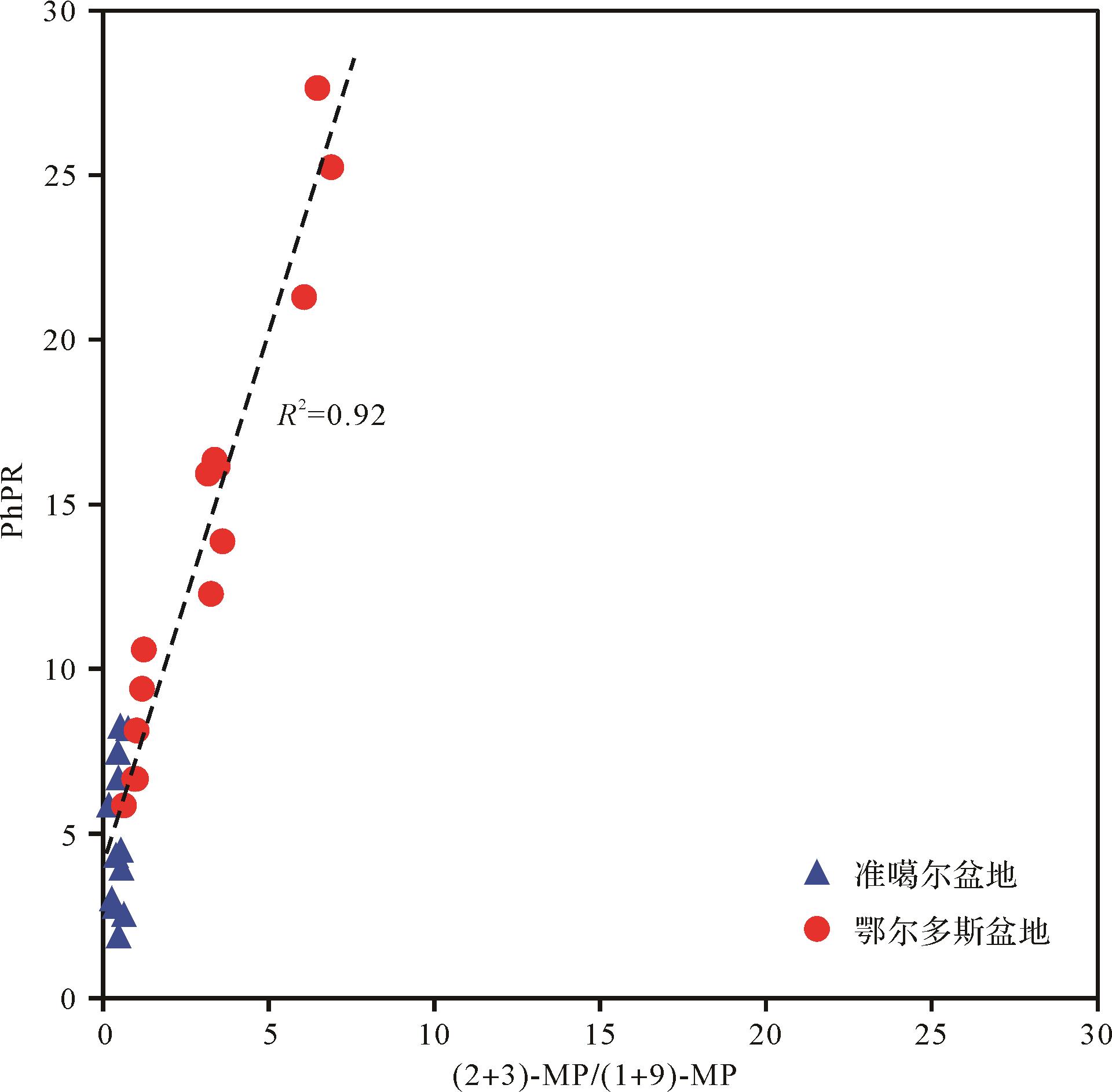
Szczerba et al.[25]通过量子化学计算和分子模拟的方法,研究了甲基菲的反应机理,认为在地质条件下甲基菲不同异构体的形成和转化通过甲基迁移作用、甲基化作用及去甲基化作用三种方式完成,其中甲基迁移作用是控制甲基菲分布的主要反应途径。1-MP通过1-,2-甲基迁移转化成2-MP很容易发生,3-MP与2-MP之间转化较容易,1-MP与9-MP之间的转化由于反应能垒较高,很难发生。为了比较苯基菲和甲基菲在反应机理上的差异性,本文将苯基菲比值PhPR与其对应的甲基菲参数((2+3)-MP/(1+9)-MP)做相关图。从图6中可以发现,甲基菲比值和其对应的甲基菲参数表现出良好的线性正相关关系,相关系数为0.92,表明苯基菲的分布可能受控于与甲基菲相似的化学反应机理,即苯基菲的生成和转化可能主要通过苯基迁移作用,随着成熟度的升高,1-PhP通过1-,2-苯基迁移作用转化为2-和3-PhP。

Figure 6. Correlation of PhPR with (2+3)⁃MP/(1+9)⁃MP for the coals from the Junggar and Ordos Basins
Rospondek et al.[30]利用量子化学计算和分子模拟的方法研究了苯基二苯并噻吩(PhDBT)系列的苯基迁移作用,1-PhDBT→2-PhDBT→3-PhDBT→4-PhDBT的反应能垒分别为13.3 kcal/mol、20.2 kcal/mol、16.2 kcal/mol。Yang et al.[24]计算了甲基二苯并噻吩(MDBT)系列的甲基迁移作用,1-MDBT→2-MDBT→3-MDBT→4-MDBT的反应能垒分别为18.7 kcal/mol、24.3 kcal/mol、20.7 kcal/mol。对比可以发现,在相同的取代位之间,苯基在二苯并噻吩上迁移需要克服的反应能垒比甲基的更小。同时,随着成熟度的增加,苯基菲比值比其对应的甲基菲参数变化更快(图6),反映苯基菲之间的转化可能比甲基菲更容易。另外,量子化学计算结果表明苯基菲比甲基菲的偶极矩(Debye)更小,其中1-PhP为极性分子,2-、3-PhP为非极性分子(表2),基于图6的结果认为随着热作用增加,极性分子更容易向非极性分子转化。而1-、2-、3-MP都为极性分子,且2-、3-MP的极性更大,1-MP向2-、3-MP转化需要更多的能量。由此可见,由于苯基比甲基在菲上的迁移需要克服的反应能垒更小,导致苯基菲比值对成熟度的变化更敏感。
3.1. 苯基菲的鉴定
3.2. 苯基菲和甲基菲异构体热稳定性差异
3.3. 成熟度对苯基菲分布的影响
3.4. 沉积环境对苯基菲分布的影响
3.5. 苯基菲和甲基菲分布对比
-
(1) 系统准确地鉴定了准噶尔盆地和鄂尔多斯盆地26件煤中苯基菲系列化合物,确定了其具体的流出位置和顺序为4-、9-、1-、3-、2-苯基菲,精确计算了苯基菲系列的热稳定性顺序为2->3->9->1->4-PhP,热稳定性差异可能是由空间位阻效应导致的。
(2) 基于苯基菲的分布特征和热稳定性量子化学计算,提出了成熟度参数PhPR,初步建立了与Ro的定量关系式:Rc(%)=0.06×PhPR+0.32(Ro>0.4%),并发现苯基菲比值受沉积环境的影响很小,该指标能够定量评价低热—高热原油和烃源岩(II~III型干酪根)成熟度。
(3) 与甲基菲分布特征和热稳定性对比,苯基菲比值的变化可能受控于苯基迁移作用,随热演化程度的增加,1-PhP通过1-,2-苯基迁移作用转化为2-和3-PhP,相对于甲基菲参数((2+3)-MP/(1+9)-MP)苯基菲比值对成熟度的变化更敏感。










 DownLoad:
DownLoad: